Vendor oversight is an important element, and a regulatory requirement, of all clinical trials just as proper communication is essential to any healthy working relationship. Many sponsors outsource some or all of the clinical trial responsibilities to a CRO, but the quality and integrity of the trial data always resides with the sponsor, so vendor oversight and quality assurance are essential.
Inefficient communication is one of the most common challenges that sponsors report when evaluating their relationships with their CROs, but it turns out this is not a one way street; CRO’s also credit communication lapses on the sponsors’ end as a paramount area of concern.
In this white paper, we discuss the importance of creating a vendor oversight plan and provide some tips and tricks for effectively communicating with your CRO.
Poor Communication: A Headache for both Sponsors and CROs
A recent survey conducted by Applied Clinical Trials asked both CROs and sponsors to identify the factors that commonly stand in the way of efficient collaborations, and both parties cited communication issues as a primary source of concern.
The main communication roadblocks for sponsors included a lack of transparency surrounding budgets, feeling disconnected from the sites and receiving incomplete information from their CROs. In essence, being “kept out of the loop.”
Interestingly, 23% of CRO respondents also reported communication issues originating on the sponsors’ end, such as insufficient or untimely responses, as well as micromanagement as major sources of frustration.
In sum, poor communication- In some cases too little, in others, too much- seems to be a significant barrier to successful CRO-Sponsor relationships. Fortunately, a thorough and detailed vendor oversight plan can remedy some of these issues and mitigate negative consequences that could potentially impact the quality of a data.
Components of Effective Vendor Oversight Plan
An effective vendor oversight plan should act as a roadmap for the sponsor and CRO, clearly defining the roles, responsibilities and expectations of each party, facilitating a positive relationship and ensuring the quality of the work it provides.
However, it is important not to confuse oversight with micromanagement – the objective of an oversight plan is to support the CRO in fulfilling their responsibilities, meeting milestones and adhere to timelines, while always leaving the lines of communication open.
Bellow are a few vendor oversight essentials, outlined in an article by Clinical Leader:
- clearly define critical processes and associated roles, responsibilities, and expectations
- know and understand agreements, scope, reporting obligations, and quality expectations
- agree on a team communication plan, including an escalation plan
- establish positive working relationships through open and honest communication
- communicate concerns, issues, and changes early to avoid surprises.
The last five years have seen a flurry of life sciences mergers and acquisitions, and the trend is expected to continue, which makes implementing a CRO oversight plan particularly essential today.
As Clinical Leader notes, “not only is vendor management critically important to FDA, but in the case of an M&A, a plan supports the ongoing performance assessment of the CRO.” Furthermore, you can update or make changes to the plan if additional oversight of a functional area or service is needed.
Metrics for Measuring Poor Communication
That brings us to perhaps the most important aspect of a vendor oversight agreement –– establishing a way to measure poor communication.
There are several ways to do this, but we recommend selecting some common quantifiable pain points, such as:
- The number of missed deadlines due to poor communication
- Error rates in data QC due to unclear specifications
- Changes in processes or team members
- Emails, phone calls, or messages still waiting for responses
If you can identify and measure the risks caused by poor communication, you can better mitigate them and stay on track.
Including Communication In Your Risk Registers
Now that you know some of the ways poor communication impacts clinical trials, consider including those challenges in your risk registers.
Poor communication is just as big of a risk as safety, supply management, and data handling concerns. By including communication risks in your risk registers, you can prevent problems from worsening and build a stronger relationship with your CRO.
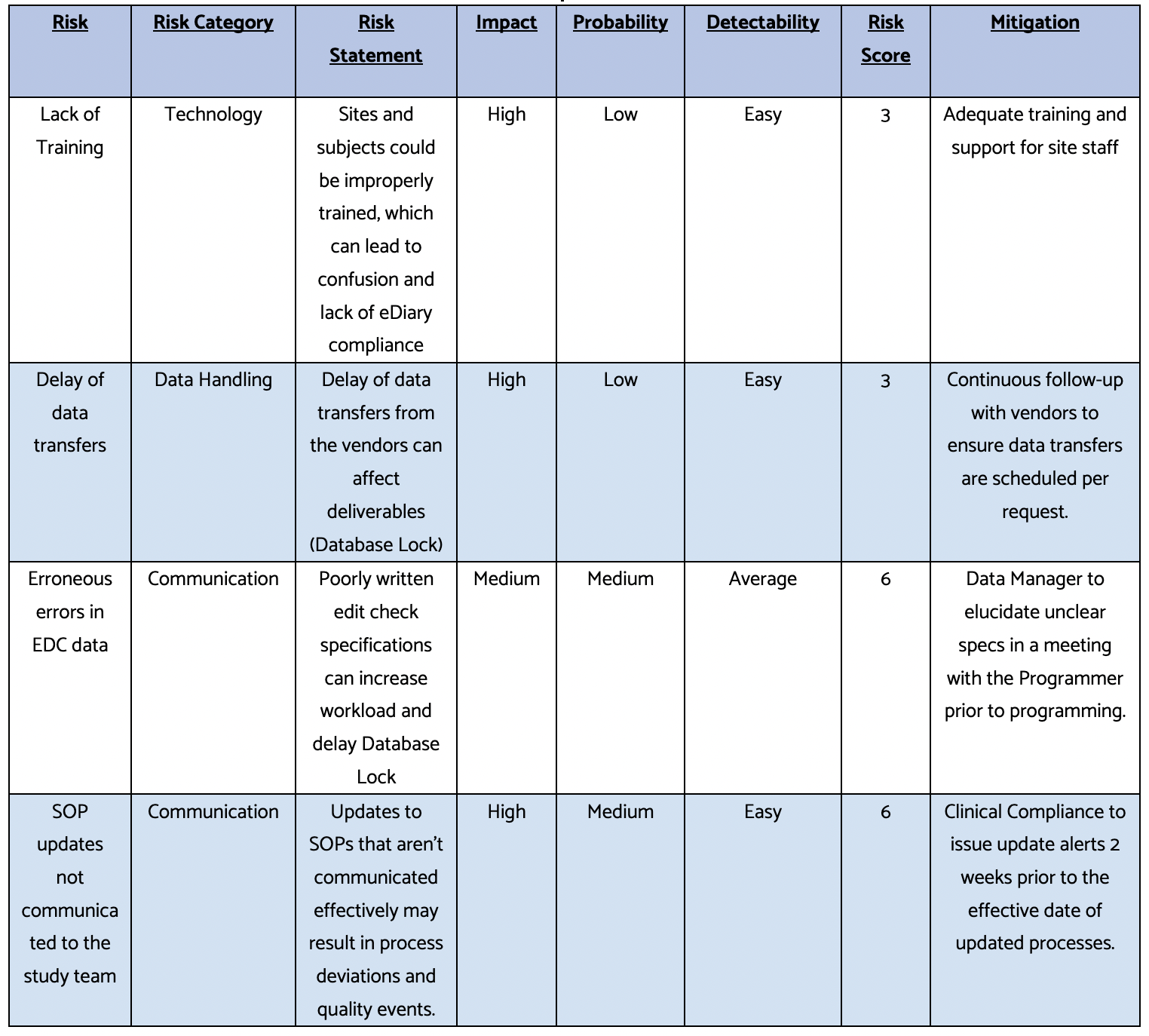
Here’s a sample risk register to help you get started:
Two-Way Communication: The Key to Cultivating Successful Sponsor-CRO Relationships
A successful conversation always has more than one participant. It’s a back and forth. A mutual give and take. Yet, this type of two-way communication is often overlooked in the clinical trial process.
The easiest way to build a strong and trustworthy relationship with your CRO is to establish and maintain open lines of communication. Scheduling routine calls or in-person meetings presents an opportunity to check in, make necessary adjustments, and ensure you’re in compliance.
Ready to take the next step?
Harbor Clinical: The Conduit of Communication
As a vendor oversight provider, we serve as a conduit of effective communication between our clients and their vendors. Our CRO/vendor oversight services keep outsourcing costs reasonable, avoiding costly CRO change orders, managing timelines, and ensuring quality is maintained.
We regularly work with biopharma companies of all sizes, providing the following vendor oversight services:
- Formalized oversight
- Established governance
- Vendor Oversight Plan development, maintenance, and management
- Measure and analyze Key Performance Indicators (KPIs)
- Maintain effective two-way communication
Interested in learning more? Take the first step toward improved vendor oversight today.
Our subject matter experts can help you stay compliant, enhance performance, and eliminate inefficiencies. Click here and fill out an online contact form or call (781) 775–0342 today.